Cryometry
Molality - Molarity
These terms describe the molecular concentration of all molecules being effective in a solution in the chemical area. Molarity is the molecular concentration in relation of the volume of a solvent. Molality is related to the mass of a solvent. With Löser - Cryometers you will measure Molalities.Molale Pressure
In practice the molality is a measure for the total number of all soluted particles in a solution. The molality m of a given solution is defined as follows: m = (1000 x u x Nmol) / (N0 x M0) with u = 1 if the solution is undissociated, otherwise u is correlating to the total number of ions are existing or may have formed in a molecule of solution. Nmol = Number of moles of undissociated substance. N0 = Number of moles of solvent, M0 = Molecular weight of solvent.Molality is measured in mol, very common in millimol (mmol). If not otherwise described the molality is measured by determining the freezing point depression. The freezing point depression and the molality are related by this formula:
Measuring Principle
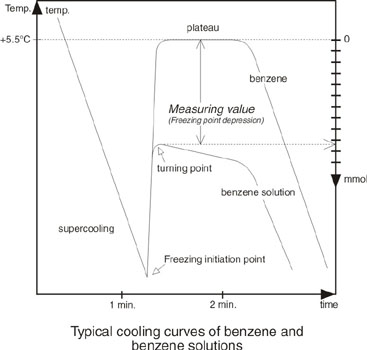
The freezing point of benzene solutions is measured. The freezing point depression in comparison to pure benzene is a direct measure of the molale concentration. Pure benzene freezes at +5.5°C; an benzene solution with an molality of 1 mol / kg benzene freezes at +0.38°C. 1 Mol of a substance dissolved in 1 kg of benzene gives a solution with a molale concentration of 1 mol / kg benzene only if it is an ideal solution and if the substance does not dissociate.Function and Description
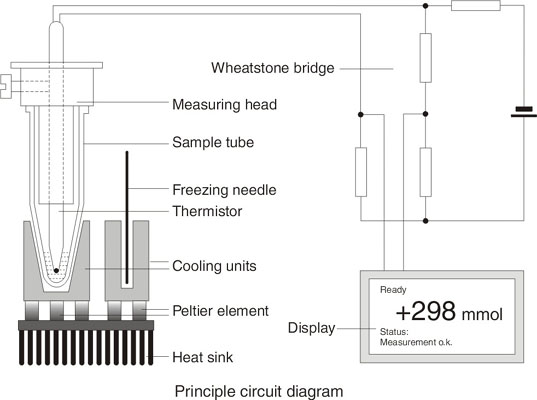
The Löser-Cryometer transports the warmth via cooling surfaces into the atmosphere, an extra water supply is not necessary. Operation of the instrument requires only an electrical socket. The temperature on the cold side is kept electronically constant.
During the measuring process the temperature of the sample is measured by a thermistor (a temperature dependent resistor). This is part of the measuring head onto which the tube is placed. The measuring head is attached to guide rods which protect it from accidental damage.
At a defined supercooling the freezing process is started by lowering a needle with ice crystals into the sample tube. The freezing point of the sample is reached. The method of initiating the freezing process is important for the reproducibility of measurements. Dipping a needle with ice crystals into the sample gives more exact results than stirring with a wire which is constantly dipping into the sample.
Because of linear correlation between molality and freezing point the measurement of freezing point is a determination of molality. The results are displayed as mmol / kg benzene.
Determination of molecular weight of unknown samples
An exact weight of the unknown sample (i.e. Oil) must be solved in a certain amount of benzene. It should be the same which was used for the zero point and the calibration solution. 150 µl of this sample solution must be pipetted into a sample tube in order to measure the molal concentration. The result shall be found in the range of 100 ... 400 mmol/kg benzene. If not it is necessary to prepare the solution again with a lower concentration. The molecular weight can be calculated by using the following equation:Field of Applications for Cryometer
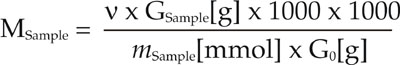
with u = 1 if the solution is undissociated like benzene, otherwise
u is correlating to the total number of ions are existing or may
have formed in a molecule of solution.
MSample = Molecular weight of unknown sample in g/mol.
GSample = Weight of unknown sample in g.
mSample = Measured molality of unknown sample in mmol.
G0 = Weight of solvent in g.